Abstract
Introduction: EBV‐positive (EBV+) diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS) often shows an aggressive clinical course with frequent extranodal disease including gastrointestinal involvement. However, the clinicopathological and prognostic significance of the EBV+ disease among primary gastric DLBCL (gDLBCL) has not been confirmed. Recently targeted therapies using antibodies against programmed cell death 1 (PD-1) and its ligand (PD-L1) have shown great promise in the treatment of relapsed or refractory DLBCL, but there remains a need for reliable methods to identify the candidates for novel immune-therapies.
We analyzed the clinicopathological characteristics of gDLBCL and shed the light on the EBV harboring on tumor cells and PD-L1 expression as prognostic indicators for the future clinical trials of PD-1 blockade.
Patients and methods: A total of 157 patients previously diagnosed as primary gDLBCL and treated with rituximab-containing chemotherapies were retrospectively examined. All cases were tested for EBV-encoded small RNA (EBER) by in situ hybridization (ISH). The expression of PD-L1 was immunohistochemically evaluated (clone SP142, Spring Bioscience, Pleasanton, CA).
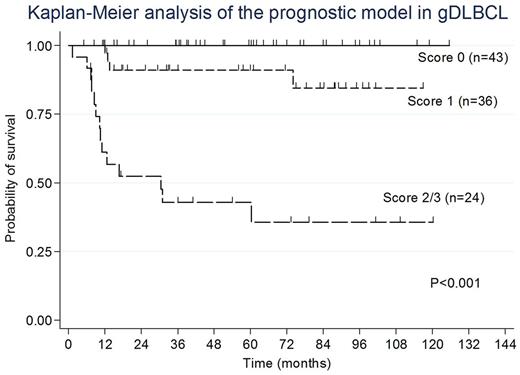
Results: Our series consisted of 157 patients: 92 males and 65 females (male: female ratio = 1.4), with a median onset age of 67 years (range, 32-89 years). 66 patients (42%) showed Lugano stage Ⅱ2/ⅡE/Ⅳ, 59 (40%) with high levels of soluble interleukin -2 receptors (sIL-2R >1000U/ml), 45 (29%) with IPI HI/H, and 34 (22%) with B symptoms. 39 patients (38%) had multiple gastric lesions, 19 (12%) with the presence of Bulky mass, and 29 (60%) with Helicobacter pylori infection. 92 patients (61%) had a non-germinal center B-cell immunophenotype (non-GCB). All of 157 patients received multiagent chemotherapy combined with rituximab, with an additional irradiation in 55 (35%). EBV harboring was found on the tumor cells in 12 (7.6%). There was no significant difference in the clinicopathological findings between EBV+ and EBV‐negative (EBV−) subgroups. But, notably, the EBV+ patients had a significantly worse overall survival (OS) than EBV− ones (P=0.004). Multivariate analysis of survival revealed that EBER positivity (P=0.012), the presence of multiple gastric lesions (P=0.014), and IPI HI/H ( P=0.017) were adverse independent prognostic factors in gDLBCL. We constructed a prognostic model by combining these prognostic variables as follows: patients with a score of 0 (n=43), no adverse factors; patients with a score of 1 (n=36), one factor; and patients with a score of 2/3 (n=24), two or three factors. The 5-year OS rates for these 3 groups were 100%, 91%, and 43%, respectively. Furthermore, this model significantly stratified gDLBCL patients separately by survival (P <0.001, Figure). Although none of gDLBCL cases expressed PD-L1 on tumor cells, 7 (78%) of 9 cases with EBV+ gDLBCL were positive for PD-L1 in ≥20% of nonmalignant stromal cells in microenvironment (miPD-L1+), which showed a higher frequency than in EBV− ones (78% vs 42%, P=0.13).
Conclusions: EBV+ gDLBCL showed a significantly worse prognosis. Our prognostic model appeared to well define the clinical outcome of gDLBCL patients. PD-L1 expression in nonmalignant stromal cells was a common feature of EBV+ gDLBCL. They could be good candidates to benefit from anti-PD-1/PD-L1 therapy. A novel prognostic model by integrating EBV harboring on tumor cells may serve as a risk‐stratification for future clinical trials in which high‐risk patients can be treated with newly developing immune-therapies to improve their outcomes.
Shimada: Kyowa Hakko Kirin Co., Ltd.: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Eisai Co., Ltd.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal